
Designated as one of the WOAH Reference Laboratory for Rabies at the 80th WOAH General Session in May 2012.
Dr. Dong-Kun Yang
[Animal and Plant Quarantine Agency]
Viral Disease Division
[Animal and Plant Quarantine Agency]
The rabies research laboratory of the Animal, Plant and Fisheries Quarantine and Inspection Agency (APQA) is designated as the national reference laboratory for rabies as well as other diseases affecting the nervous system and provides technical support for the national rabies eradication programme. The rabies research laboratory is developing several kinds of diagnostic method using advanced techniques such as immunodiagnostic kit, RT-PCR kit, monoclonal antibody and new ELISA kit.
The FAT is performed according to the procedure described in the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. The frozen thin sections of the Ammon's horn tissue are fixed on the slides with cold acetone (-20℃) for 20 min. After three successive washing with phosphate buffered saline (PBS, pH 7.2), the slides are reacted with specific monoclonal antibody (JenoBiotech, Chuncheon, Korea) against rabies virus for 45 min at 37℃, and then stained with fluorescence isothiocyanate (FITC) conjugated goat-anti mouse IgG+IgM. After rinsing with PBS, the slides are air-dried and mounted with buffered glycerin (Southern Biotechnology Associate, Birmingham, USA). The slides are examined under cover slips at 400X using a fluorescent microscope (Nikon, Tokyo, Japan). Positive and negative controls are run together with the test samples. The slide showing specific fluorescence is confirmed as positive.
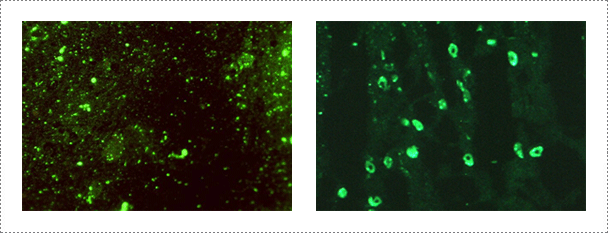
Fig. 1. Detection of rabies virus by indirect fluorescent antibody test in infected brain samples (Journal of Veterinary Science, Yang DK et al., 2012).
Samples are submitted from provincial and private laboratories throughout Korea. APQA routinely conducts virus isolation from the submitted samples by using neuroblastoma cell lines (CCL-131 obtained from the American Type Culture Collection: ATCC) according to the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals at a bio-safety level (BSL) II plus facility located at the rabies laboratory. Virus isolation samples include brain obtained from rabid domestic and wild animals. For the past three-years, the rabies laboratory of APQA has tested a total of 150 samples for virus isolation using neuroblastoma cell lines. The diagnostic tests used for virus identification include indirect fluorescent antibody test (FAT), virus isolation, specific and rapid immunochromatographic (IC) assay for RABV and reverse transcription-polymerase chain reaction (RT-PCR).
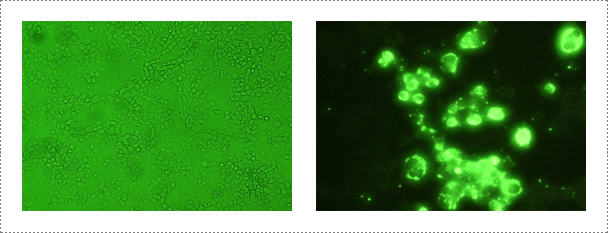
Fig. 2. Rabies virus identification in NG108-15 cell. Mouse neuroblastoma cells are routinely used for the isolation and identification of rabies virus (Journal of Veterinary Science, Yang DK et al., 2011, accepted)
The commercial rapid immunodiagnostic assay (RIDA) kit is used according to the manufacturer's instruction. A 10% brain suspension of each sample is prepared and a swab supplied with the kit is dipped in the brain homogenate. The swab is transferred to the solution for virus extraction. A 100 μl aliquot of the samples is dispensed into the sample well. After a 5 min reaction time, the results are read and the appearance of two lines is considered to be positive.
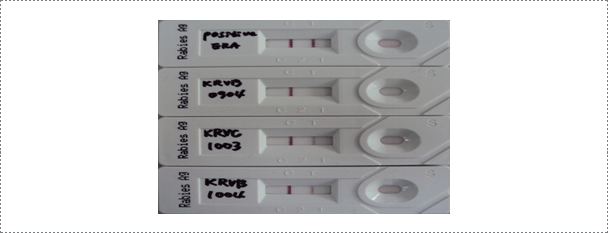
Fig. 3. Results of the rapid immunodiagnostic assay (RIDA) after applying samples (ERA strain, wild type RABVs: KRVR0904, KRVC1003 and KRVB1004) (Journal of Veterinary Science, Yang DK et al. 2012).
Viral RNA is extracted from brain samples using an RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction. The extracted RNA is eluted in 50 μl of RNase- and DNase-free water. RT-PCR is carried out for the detection of RABV genome using specific primer set (RVNDF and RVNDR) that amplify the N gene of RABV (Table 1). The RT-PCR was performed in a reaction mixture containing 5 μl of denatured RNA, 1 μl of each primer (50 pmol), 5 μl of 5X buffer (12.5 mM MgCl2), 1 μl of dNTP mix, 1 μl of enzyme mix (reverse transcriptase and Taq polymerase), and 11 μl of distilled water (Qiagen, Hilden, Germany). The cycling profile is as follows: cDNA synthesis at 42°C for 30 min, followed by 45 cycles of 95℃ for 15 sec, 55℃ for 15 sec, and 72℃ for 15 sec, with a final extension at 72℃ for 5 min. The RT-PCR products are visualized using electrophoresis on 1.8% agarose gels containing ethidium bromide. The rabies research laboratory routinely performs RT-PCR using one step RT PCR kit to determine whether samples contain RABV. Currently, the RT-PCR kit is also routinely used for rapid detection of RABV by the provincial diagnostic laboratories as supplementary diagnostic method. For the past three years, the rabies research laboratory has performed RT-PCR assay for approximately 200 samples.

Fig. 4. Reverse-transcription polymerase chain reaction (RT-PCR) assay for virus identification. The RABV laboratory routinely conducts RT-PCR assay for virus identification and characterization of RABV.
Table 1. List of the oligonucleotide primers used for RT-PCR of the rabies virus
| Primer | Nucleotide sequences (5'-3') | Nucleotide position* |
Sense | RABV gene |
Size of amplicon (bp) |
|---|---|---|---|---|---|
| RVNDF | GRA ATT GGG CTT TGA CTG GA | 353-372 | + | N | 181 |
| RFNDR | AAA GGG GCT GTC TCG AAA AT | 514-533 | - |
*: The positions of primers are based on the ERA strain (GenBank accession no. AF406695, AF406693).
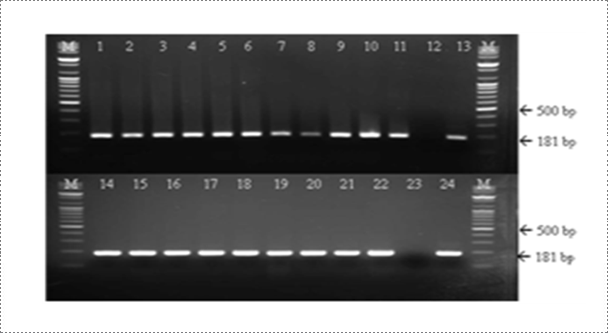
Fig. 5. Detection of rabies virus (RABV) by RT-PCR assay. A common type PCR primer set (product size of 181 bp) was designed to detect all RABV. The rabies laboratory routinely conducts RT-PCR assay for rapid detection of virulent RABV (Journal of Veterinary Science, Yang DK et al, 2012).
Mouse inoculation tests are performed according to the procedure described in the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. A 20% brain suspension of each sample is prepared and 0.03 ml is inoculated intracerebrally in 5 to 10 young white Balb/C mice (21-25 days old) weighing 11-15 g. The animals are checked daily within 30 days post-inoculation for typical rabies symptoms, such as emaciation, tremors, lack of coordination, paralysis and death. The mean survival time of the inoculated mice is defined as the mean time after inoculation between the day of the first appearance of clinical signs and the day of death.

Fig. 6. An animal experiment for determining the virulence of RABV isolates. Intracerebral experiment using specific pathogen free mice is one of methods used at the RABV laboratory for checking the virulence of RABV isolates. All experiments are performed in a BL 3 experimental animal facility.
Table 2. Sensitivity and specificity of VI, RT-PCR and RIDA in relation to FAT for the detection of rabies virus using field brain samples¹
| VI | RT-PCR | RIDA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P* | N | Total | P | N | Total | P | N | Total | ||
| FAT | P | 20 | 0 | 20 | 20 | 0 | 20 | 19 | 1 | 20 |
| N | 0 | 90 | 90 | 0 | 90 | 90 | 0 | 90 | 90 | |
| Total | 20 | 90 | 110 | 20 | 90 | 110 | 19 | 91 | 110 | |
| Sensitivity (%) | 100 | 100 | 95 | |||||||
| Specificity (%) | 100 | 100 | 98.9 | |||||||
*P: positive, N: negative. ¹VI, virus isolation; RT-PCR, reverse transcriptase polymerase chain reaction; RIDA, rapid immunodiagnostic assay; FAT, fluorescent antibody test (Journal of Veterinary Science, Yang DK et al., 2012).
As shown in Table 2, sensitivity of VI, RT-PCR and RIDA was shown to be 100, 100 and 95% compared with FAT results and these results were similar to that of the previous reports. The KRBV0904 isolate was positive by VI, RT-PCR and FAT, but negative by the RIDA kit (98.9% specificity). The false negative result by the RIDA kit may be due to the kit's low detection limit compared with the other diagnostic methods described here. The false-negative KRVB0904 sample had a viral titer of 101.5TCID50/ml, which was too low to be detected by the RIDA kit. It has been reported that the detection limit of RT-PCR was at 100.5LD50/0.03ml using the N gene of RABV, which was much lower than that of the RIDA kit (101.7LD50/0.03ml). Based on the results of this study, VI and RT-PCR revealed to be highly sensitive and specific detection methods for RABV. Despite of the comparatively low sensitivity, RIDA kit is the best option for a rapid and easy to perform rabies virus detection test. In conclusion, with the combined diagnostic methods according to the laboratory situation, it is possible to use these kinds of diagnostic methods and to make a rapid decision for controlling rabies.
Rabies-neutralizing antibodies could be detected by the FAVN test. The WOAH serum of dog origin or a positive reference serum from dog origin adjusted to 0.5 IU/ml is used as a positive control. Briefly, each serum sample as well as the positive and negative controls were distributed in four consecutive wells, and then serially diluted. Fifty µl of the challenge rabies virus (CVS-11, ATCC VR-959) containing a titer of 100 TCID50 is then added to each well. After 60 min of incubation, 50 µl of 4 x 105 cells/ml suspension is added to each well and the microplates are incubated for 48 h at 36 ± 2 °C in a humidified incubator with 5% CO2. The microplates are stained by adding 50 µl of an appropriate dilution of a fluorescein isothiocyanate (FITC) conjugated anti-rabies monoclonal antibody to each well. Plates are qualitatively read according to an "all or nothing" scoring method. The titres of serum samples are expressed in International Units per millilitre (IU/ml) by comparing results obtained with those of the positive standard. The threshold of positivity used is 0.5 IU/ml.
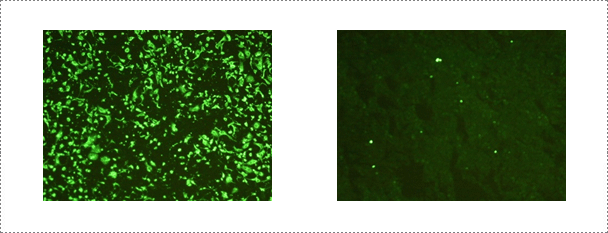
Fig. 7. Serological test performed at the rabies laboratory. Fluorescent antibody virus neutralization (FAVN) test is conducted routinely at the rabies laboratory for serology of rabies.
The ERA RABV strain (previously used as a vaccine strain in Canada and South Korea) is used in the neutralizing peroxidase-linked assay (NPLA). The NPLA is performed in 96-well microplates. Serum samples are inactivated for 30 min at 56℃, serially diluted (50 µl) two-fold in α-MEM (50 µl), mixed with an equal volume of 100-200 FAID50 (Fluorescent assay infectious dose) of the ERA strain, and incubated at 37℃ for 1 h. Vero cells (100 µl) are added to each well at a concentration of 2 x 104 cells in α-MEM containing 10% fetal bovine serum. Microplates are incubated for 5 days at 37℃ in a 5% CO2 incubator. Plates are washed once with PBS and fixed with 80% cold acetone for 15 min. Anti-RABV mAb is added to each well and incubated for 40 min at 37℃. After washing, anti-dog HRP conjugate is also added to each well and incubated for 40 min at 37℃. Plates are washed three times and then stained with chromogen substrate solution 3', 3-diaminobenzidine (Sigma) and hydrogen peroxidase for 30 min at 37℃. Serum titers are recorded as the reciprocal of the highest initial dilution of sera that neutralized rabies virus replication in 100% of the wells. Serum samples demonstrating VN titers greater than 1:2 are considered positive and greater than 1:16 were considered protective to wild-virus infection.
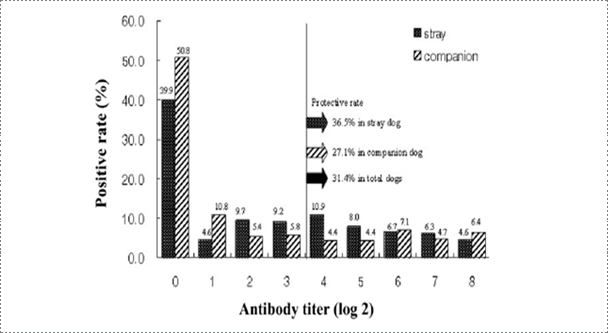
Fig. 8. Frequency distribution of antibody titers from stray and companion dogs of Korea (Korean J Vet Res, Yang DK et al., 2010).
Rabies research laboratory estimated the nationwide rabies immune status of stray and companion dogs through NPLA testing of canine sera obtained from rescue centers and veterinary clinics. According to the previous reports, Rabies seroprevalence in stray dogs was estimated to be 27.7% in Japan and 62% in Thailand. It was reported that 35% of dogs near the Pukhansan National park and Seoul city were seropositive against rabies. The average prevalence of antibody against rabies was 54% in the present study considering that the detected antibodies were induced by vaccination. Based on the result of this study, the positive rate in Korean stray dogs was higher than that of Japan and lower than that of Thailand. In addition, the positive rate in the stray dogs was greater than that in companion dogs, suggesting that some stray dogs may have been initially reared as pet animals. The serological data analysis demonstrated that the regional prevalence rate ranged from 30.4% to 87.5%. The seropositive rate in the Gangwon province was very low, suggesting that the low detection rate of rabies antibody could be correlated with rabies outbreaks in the Gangwon province, contact with raccoon dogs as carriers of rabies and domestic animals could increase the incidence of rabies.
The Platelia™ Rabies II kit ad usum Veterinarium along with the reagents of the ELISA kits were obtained from Bio-Rad.

Fig. 9. Serological test is being performed at the rabies laboratory. ELISA test is one of the diagnostic assays that are routinely conducted in the laboratory for the determination of antibodies against the rabies virus.
After serum samples are diluted with dilution buffer, the samples are added to each wells. Three washings are performed after incubation to remove unbound antibodies and other proteins in the samples. Then, 100 µl of the conjugate (protein A labelled with peroxidase) is added to each well. The microplates are incubated for 1 h at 37°C. Five washings are performed after incubation to remove unbound conjugate. The presence of immune complex is revealed by adding 100 µl of TMB chromogen solution to each well. The microplates are incubated in the dark for 30 min at room temperature. The enzymatic reaction is stopped by adding a solution of 1 N H2SO4. The microplates are read bi-chromatically at 450 and 620 nm. The calculation of the titer in ELISA units (EU)/ml is done using the method provided by the manufacturer on a CD included with the kit. The threshold of positivity is 0.5 EU/ml.